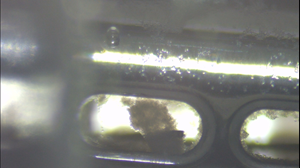
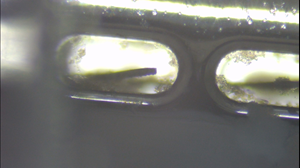
Recent Study Validates the Operational Effectiveness of Microbot Medical’s Self-Cleaning Shunt (SCS™)
HINGHAM, Mass.,
|
||||||||||||||||||||
“We believe the highly encouraging results from this latest study, which complements our previous pre-clinical studies, demonstrates the potential of our SCSTM product to revolutionize how Hydrocephalus and Normal Pressure Hydrocephalus (NPH) can be treated in the future,” commented Harel Gadot, CEO, President and Chairman. “We believe the performance of the SCSTM during this and previous studies gives us greater confidence to explore additional medical applications for the device in conditions where occlusion occurs, such as in the Traumatic Brain Injury (TBI) space.”
The study was conducted at Envigo CRS Israel, a leading provider of non-clinical contract research services and research models. Human brain glioblastoma cells were used in order to assess performance of the SCSTM in a test system with accelerated cell growth rate, accumulation and obstruction rates. The study commenced in
- Significant cell growth and accumulation in a non-operating SCSTM; and
- A significant inhibition in cell growth in the constantly operating SCSTM with very little to no cell attachment on the robotic brush (ViRobTM) and on the opening where the robotic brush (ViRobTM) operates.
- The study also demonstrated that the Company’s SCSTM has the ability to operate after cells had accumulated on the catheter holes and the robotic brush (ViRobTM).
Moreover, SCS TM activation demonstrated the potential to disintegrate existing occlusions formed on the robotic brush (ViRobTM) and on the opening where the robotic brush (ViRobTM) operates.
In addition to this study, the Company previously announced data from two pre-clinical studies that were performed at leading U.S. academic institutions. Data from those studies were also presented by Professor Pat McAllister,
- In-vitro study, which was performed at
Wayne State University , supports the SCSTM’s potential as a viable technology for preventing occlusion in shunts used to treat hydrocephalus. - In-vivo animal study, which was performed at
Washington University School of Medicine in St. Louis, supports the safety profile of the Company’s SCSTM as a CSF catheter.
The follow up study, which is being conducted by the same academic institutions, commenced in
About Envigo
With over 3,300+ employees serving over 65 countries with a network of more than 25 operating facilities worldwide, Envigo provides comprehensive scientific expertise and a full service offering in non-clinical research and development, research models and services, regulatory consulting, and analytical support to our customers. Envigo is a privately held global company with corporate headquarters in New Jersey
About
Microbot™, which was founded in 2010 and commenced operations in 2011, became a NASDAQ listed company on
The ViRobTM technology is a revolutionary autonomous crawling micro-robot which can be controlled remotely or within the body. Its miniature dimensions allow it to navigate and crawl in different spaces within the human body, including blood vessels, the digestive tract and the respiratory system. Its unique structure gives it the ability to move in tight spaces and curved passages as well as the ability to remain within the human body for prolonged time. To learn more about ViRobTM please visit http://www.microbotmedical.com/technology/virob/.
TipCATTM is a transformational self-propelled, flexible, and semi-disposable locomotive device providing see & treat capabilities within tubular lumens in the human body such as the colon, blood vessels, and the urinary tract. Its locomotion mechanism is perfectly suitable to navigate and crawl through natural & artificial tubular lumens, applying the minimal necessary pressure to achieve the adequate friction required for gentle, fast, and safe advancement within the human body. To learn more about TipCATTM, visit http://www.microbotmedical.com/technology/tipcat/.
CardioSertTM technology contemplates a unique combination of a guidewire and microcatheter, technologies that are broadly used for endoluminal surgery. The CardioSertTM technology features unique steering and stiffness control capabilities, and it was originally developed to support interventional cardiologists in crossing the most complex lesions called chronic total occlusion (CTO) during percutaneous coronary intervention (PCI) procedures and has the potential to be used in other spaces and applications, such as peripheral intervention, neurosurgery and urology. CardioSertTM was part of a technological incubator supported by the Israel Innovation Authorities (formerly known as the
Statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities for
Investor Contact:
Michael Polyviou
EVC Group
mpolyviou@evcgroup.com
732-933-2754
Photos accompanying this announcement are available at
http://www.globenewswire.com/NewsRoom/AttachmentNg/0e66d290-1850-4767-9463-2244dc378263
http://www.globenewswire.com/NewsRoom/AttachmentNg/685a77ab-f5b4-47c2-86a5-2844464eb363
http://www.globenewswire.com/NewsRoom/AttachmentNg/e804e02f-f20a-4ad3-bb93-d41648336b2c