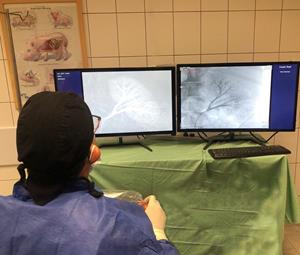
World’s First Fully Disposable Surgical Robot Achieved all Endpoints with No Intraoperative Adverse Events
Remotely Performed Procedures have the Potential to Reduce Radiation Exposure, Physical Strain and Cross Contamination to Both the Patients and the Surgical Staff
HINGHAM, Mass., Aug. 17, 2020 (GLOBE NEWSWIRE) -- Microbot Medical Inc. (Nasdaq: MBOT), today announced the successful conclusion of its feasibility animal study using the Company’s LIBERTY™ Robotic System, the world’s first fully disposable surgical robotic system. The study met all of its end points with no intraoperative adverse events, which supports the Company’s objectives to allow physicians to conduct a catheter-based procedure from outside the catheterization laboratory (cath-lab), avoiding radiation exposure, physical strain and the risk of cross contamination. It was performed by two leading physicians in the neuro vascular and peripheral vascular intervention spaces, and the results demonstrated robust navigation capabilities, intuitive usability and accurate deployment of embolic agents, most of which was conducted remotely from the cath-lab’s control room.
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/a18d5bb2-f23e-4e1a-a7e0-945da51172c6
https://www.globenewswire.com/NewsRoom/AttachmentNg/60931858-446a-41ec-9adc-67524506eeb4
“We continue to validate the LIBERTY™ Robotic System and confirm our assertion that it has the potential to revolutionize the robotic surgery space by democratizing endoluminal procedures with enhanced clinical and economical outcomes,” commented Harel Gadot, CEO, President and Chairman. “The freedom from capital equipment, coupled with the ability to operate LIBERTY™ remotely, especially in today’s COVID-19 healthcare environment, will potentially reduce the cross contamination between the surgical staff and patients on top of reducing radiation exposure. Overall, we believe that we are in the right market, at the right time and with the right solution.”
Mr. Gadot, along with other members of the management team, will be sharing these results at the Needham Virtual Med Tech & Diagnostics Conference being held today. A copy of the investor presentation, which highlights the initial results of the feasibility animal study, can be accessed via the ‘Investors’ section, under ‘Presentation + Resources’ of the Company’s website at www.microbotmedical.com.
The Company believes that the LIBERTY™ Robotic System, which was unveiled in January 2020, is the world’s first fully disposable robotic system which features a unique compact design; With the capability to be operated remotely, it is being designed to reduce radiation exposure, the physical strain to the physician as well as cross contamination between the surgical staff and patients. In addition, incorporating the technology the Company acquired from CardioSert into the LIBERTY™ Robotic System, has the potential to eliminate the use of multiple consumables through its “One & Done” capabilities.
About Microbot Medical
Microbot Medical Inc. (NASDAQ: MBOT) is a pre-clinical medical device company that specializes in transformational micro-robotic technologies, focused primarily on both natural and artificial lumens within the human body. Microbot’s current proprietary technological platforms provide the foundation for the development of a Multi Generation Pipeline Portfolio (MGPP).
Microbot Medical was founded in 2010 by Harel Gadot, Prof. Moshe Shoham, and Yossi Bornstein with the goals of improving clinical outcomes for patients and increasing accessibility through the use of micro-robotic technologies. Further information about Microbot Medical is available at http://www.microbotmedical.com.
Safe Harbor
Statements pertaining to the registered direct offering, timing, the amount and anticipated use of proceeds and statements pertaining to future financial and/or operating results, future growth in research, technology, clinical development, and potential opportunities for Microbot Medical Inc. and its subsidiaries, along with other statements about the future expectations, beliefs, goals, plans, or prospects expressed by management, constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and the Federal securities laws. Any statements that are not historical fact (including, but not limited to statements that contain words such as “will,” “believes,” “plans,” “anticipates,” “expects” and “estimates”) should also be considered to be forward-looking statements. Forward-looking statements involve risks and uncertainties, including, without limitation, market conditions and the satisfaction of customary closing conditions, risks inherent in the development and/or commercialization of potential products, including LIBERTY™, the outcome of its studies to evaluate the SCS and other existing and future technologies, uncertainty in the results of pre-clinical and clinical trials or regulatory pathways and regulatory approvals, uncertainty resulting from the COVID-19 pandemic, need and ability to obtain future capital, and maintenance of intellectual property rights. Additional information on risks facing Microbot Medical can be found under the heading “Risk Factors” in Microbot Medical’s periodic reports filed with the Securities and Exchange Commission (SEC) and in the prospectus supplement related to the registered direct offering to be filed with the SEC, which are or will be available on the SEC’s web site at www.sec.gov. Microbot Medical disclaims any intent or obligation to update these forward-looking statements, except as required by law.
Investor Contact:
Michael Polyviou
EVC Group
mpolyviou@evcgroup.com
732-933-2754

Source: Microbot Medical